Recently, Professor Huang Jun's team from the School of Chemistry and Chemical Engineering at University of South China published a research paper titled "Total Synthesis of Lugdunomycin via Sequential Photoinduced Spiroketalization and Isobenzofuran Diels-Alder Reactions" in Angew. Chem. Int. Ed., a top-tier journal in the field of chemistry (with an impact factor of 16.1 and ranked in the first category by the Chinese Academy of Sciences).

Paper link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202422615
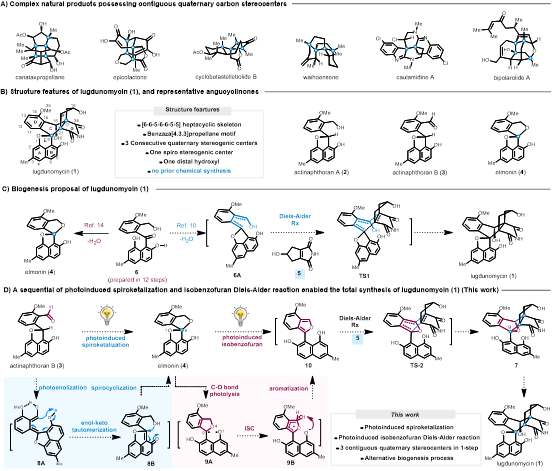
Lugdunomycin is a natural product with selective antibacterial activity against Gram-positive bacteria Bacillus subtilis 168. It features a unique [6-6-5-6-6-5-5] heptacyclic skeleton and a benzodiazepino[4,3,3]spiro[2.2]pentalene structure, and contains three consecutive quaternary carbon centers, making its total synthesis highly challenging. The innovations of this study include: (1) The development of a new method of light-induced 1,5-HAT/keto-enol tautomerization/spiroketalization to efficiently convert the natural product actinaphthoran B to elmonin. Mechanistic experiments verified that the 1,7-H shift process during the keto-enol tautomerization can occur either through the intramolecular phenolic hydroxyl group or through the proton in intermolecular water. (2) The development of a new strategy of light-induced C-O bond homolysis/inter-system crossing/1,7-proton transfer-arylation/isobenzofuran Diels-Alder reaction to construct the three consecutive quaternary carbon stereocenters and the benzodiazepino[4,3,3]spiro[2.2]pentalene of the natural product lugdunomycin. This research work completed the total synthesis of lugdunomycin through 13 steps of transformation.
Natural products have long been an important source for drug discovery. Molecules with complex spatial structures and significant biological activities, especially those with multiple consecutive quaternary stereogenic centers (CQS), have been a focus of attention. The presence of consecutive quaternary stereogenic centers in the molecular structure of natural products significantly increases the complexity and diversity of the molecules, and the special spatial structure often exhibits particular biological activities. However, the construction of consecutive quaternary stereogenic centers, especially when they exist in complex structures such as polycyclic and bridged rings, is one of the most challenging problems in the total synthesis of natural products. This strategy demonstrates the advantages of photoinduced Diels-Alder reactions in constructing multiple consecutive quaternary stereogenic centers, providing new ideas for the synthesis of other complex natural products, especially those containing multiple consecutive quaternary stereogenic centers, and showing its broad application prospects in related total syntheses of natural products.
This research was supported by the National Natural Science Foundation of China. Professor Jun Huang from the University of South China is the sole corresponding author of this article.